Abstract
Background
Cancer patients with venous thromboembolism (VTE) are at a substantial risk of recurrent VTE despite anticoagulation therapy. Limited data is available regarding the effectiveness and safety of rivaroxaban in patients with active cancer and associated VTE.
Methods
This prospective, multicenter, open-label study enrolled patients with active cancer and newly diagnosed, objectively confirmed, lower-extremity deep vein thrombosis (DVT), pulmonary embolism (PE) or both between November 2013 and June 2016. Active cancer was defined as a histologically confirmed maligancy, which was diagnosed or treated within the previous 6 months, or as a recurrent/metastatic cancer. Patients received rivaroxaban 15mg twice daily for the first 3 weeks, followed by 20mg once daily for 6 months. The primary objective of this study was to assess the symptomatic, recurrent VTE events following rivaroxaban treatment. The secondary objectives included any recurrent VTE events, bleeding complications (major or non-major clinically relevant [NMCR] bleeding events), and overall mortality. Blinded central adjudication was performed for all recurrent VTEs, bleeding events, and deaths. The recurrent VTE and bleeding events were calculated using a cumulative incidence method that incorporated death from any cause as competing risk. The study was registered at www.clinicaltrials.gov as #NCT01989845.
Results
127 patients were screened from 9 centers in Korea. Of these, 3 patients were not enrolled because they did not meet the eligibility criteria. Thus, 124 patients were enrolled. Mean age was 67 years (range, 28-85) and 55 (44%) were female. 27 patients (22%) had baseline ECOG performance status of 2. The most common primary tumor sites were lung (N=30, 24%), colorectum (N=24, 19%), pancreaticobiliary tract (N=16, 13%), stomach (N=16, 13%); 14 patients (11%) had hematologic malignancies. 93 patients (75%) had metastatic disease and 110 (89%) were under cancer treatments (chemotherapy and/or radiation). The qualifying thrombotic event was PE ± lower-extremity DVT in 102 patients (82%) and presented as symptomatic in 78 patients (63%).
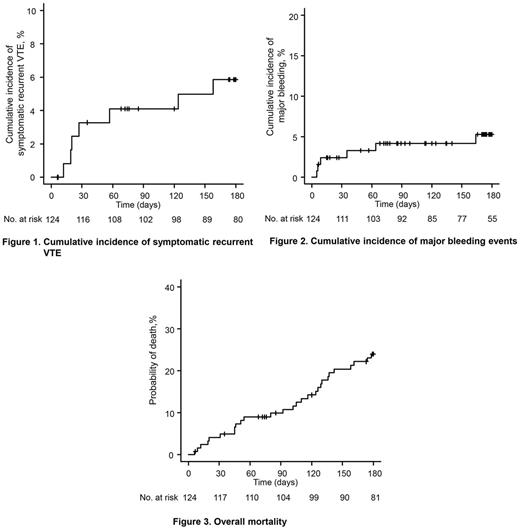
During the 6-month study period, 7 patients (fatal PE, 4; non-fatal PE, 3) experienced symptomatic recurrent VTE (cumulative rate, 5.9%, Figure 1). There were 2 patients with incidental recurrent PE. Thus, cumulative rate of any recurrent VTE was 7.6%. Major bleeding events occurred in 6 patients (cumulative rate, 5.3%; Figure 2) and NMCR bleeding events in 11 (cumulative rate, 10.2%). 28 patients died during the study period (cumulative rate, 24.0%; Figure 3).
Conclusion
Rivaroxaban appears to be an effective and safe treatment in patients with cancer-associated VTE.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal